Cell Stem Cell: Deng Hongkui’s team at Peking University establishes a more rapid and efficient human cell chemical reprogramming system
On March 20, 2023, Beijing time, Deng Hongkui's team at Peking University published a research paper entitled "Highly efficient and rapid generation of human pluripotent stem cells by chemical reprogramming" in the international academic journal Cell Stem Cell.

This study has established a new chemical reprogramming system, which can more quickly and efficiently induce human Somatic cells into pluripotent stem cells.
Pluripotent stem cells have the ability of unlimited self-renewal and differentiation into all functional cell types of organisms. These magical characteristics make them have extensive application value in cell therapy, drug screening, disease modeling, and other fields, and they are the most critical "seed cells" in the field of Regenerative medicine. How to induce and obtain pluripotent stem cells in vitro has always been a key scientific issue in the field of life sciences. In 2013, Deng Hongkui's research team published an original achievement in Science magazine, that, independent of endogenous substances such as oocytes and transcription factors, the use of exogenous chemical small molecules can reverse the fate of cells, reprogramming mouse Somatic cells into chemically induced pluripotent stem cells (CiPS cells), opening a new path for Somatic cell reprogramming. In 2022, Deng Hongkui's research team made a breakthrough and successfully induced human Somatic cells into multipotential stem cells (human CiPS cells) by using small chemical molecules (Nature, 2022).
The essence of life is a Chemical process. It is theoretically the most effective way to regulate the fate of cells through small chemical molecules. There is an essential difference between chemical reprogramming and traditional reprogramming techniques: Traditional transgenic reprogramming techniques, such as induced pluripotent stem cell technology (iPS technology), drive direct changes in cell fate through overexpression of endogenous transcription factors, and the induction process is difficult to control; Chemical reprogramming, on the other hand, utilizes exogenous chemical small molecules to simulate external signal stimuli and drive cell fate to change in a phased manner. Therefore, this method has strong controllability and is expected to achieve precise regulation of cell fate, and reversal of cell identity, and functional status, making reverse development possible.
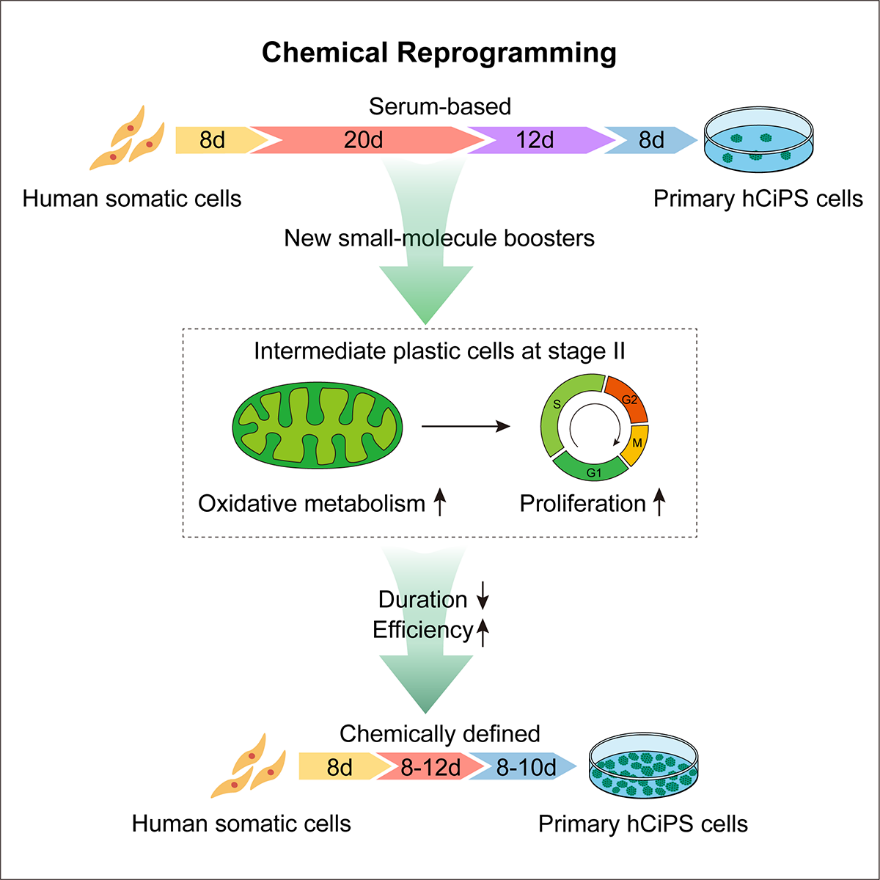
In the latest research results published this time, Deng Hongkui's research team has established a set of more rapid, efficient, and stable human cytochemical reprogramming methods. Researchers have discovered new combinations of chemical small molecules, significantly accelerating the reprogramming process, and shortening the induction cycle from 50 days to less than 30 days, with a minimum induction time of 16 days. At the same time, the induction efficiency has significantly improved, reaching up to 31%. The new system was tested on Somatic cell cells from 17 individuals of different genetic backgrounds and ages, all of which can achieve efficient induction, accelerating the pace of extensive application of human CiPS cells in cell therapy, drug screening, and disease models.
According to the previous report of Deng Hongkui's research group, the original system has experienced the epithelial-like cell stage, the plastic intermediate cell stage, and the embryonic outer Endoderm cell (XEN-like) stage in the process of inducing human CiPS cells, and finally established multipotential stem cells (Nature, 2022). This study found a more rapid and efficient molecular mechanism of the new system: plastic intermediate cells significantly enhanced their proliferation ability and Oxidative phosphorylation metabolic activity, no longer went through the XEN-like phase, and the activation of pluripotent genes was faster and the molecular path was more direct. Traditional iPS reprogramming must rely on the gradually enhanced Glycolysis metabolic process, while the production of the most critical stage of chemical reprogramming, the plastic intermediate state, relies on Oxidative phosphorylation and does not rely on Glycolysis metabolism. This discovery reveals the importance of specific energy metabolism pathways in different cell fate transformation processes, providing a new perspective for understanding the mechanisms of cell fate regulation from the perspective of energy metabolism.
A faster and more efficient human cytochemical reprogramming system was established in this study
The new induction protocol established in this study is not only fast, efficient, and stable, but more importantly, its components are chemically defined, and independent of serum-free and feeder-free cells. These attributes better meet the needs of clinical application, laying the foundation for establishing a human CiPS cell line that meets clinical application standards and making it a crucial step toward clinical application. Compared with genetically modified overexpressed transcription factors, chemical small molecules have advantages such as a non-integrated genome, reversible action, and simple operation. Therefore, CiPS technology is safer, simpler, and easier to standardize, with broad clinical application prospects. At present, Deng Hongkui's research team has efficiently prepared islet cells using human CiPS cells and has verified its safety and effectiveness in treating diabetes in large animal models, highlighting the clinical application value of human CiPS cells as "seed cells" in treating major diseases (Nature Medicine 2022; Nature Metabolism, 2023).
Professor Deng Hongkui of Peking University and Dr. Wang Jinlin are the co-corresponding authors of this research achievement. Liu Yangshijia, Wang Guan, Wang Yanglu, He Huanjing, and Lv Yulin from Peking University are the main authors of this research achievement. Professor Li Cheng from Peking University guided the bioinformatics analysis of this study.
This work was supported by the National Natural Science Foundation of China Basic Science Center, the National Key R&D Program, and the Peking University Tsinghua Life Sciences United Center.