Our Mission
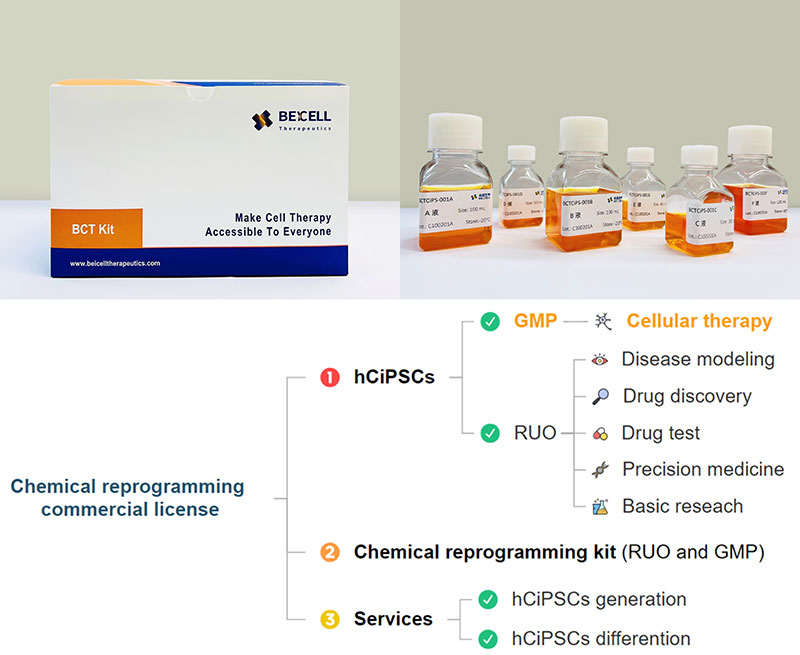
Beicell Therapeutics was established on July 1, 2022. Our core innovations are the Chemically induced Pluripotent Stem Cells (CiPSC) technology, as well as our proprietary hypoimmune platform and Xeno-free differentiation protocols. These innovative technologies enable us to develop off-the-shelf cell therapy products with high quality and low cost.
BeiCell's infrastructure includes a 40,000 sqft space with Research, Process development (PD), Analytical development (AD) labs and a Good Manufacturing Practices (GMP)-compliant facility. The co-location of these facilities allows for the seamless continuity of processes from discovery to clinical material manufacturing.
Our GMP-compliant facility is comprised of 4 ISO Class 5/Grade B clean rooms (2000 sqft), and 6 ISO 7/Grade C clean rooms (4000 sqft), capable of conducting multiple cell banking and clinical manufacturing campaigns simultaneously.

Our Team
Dr. Deng graduated from UCLA and worked as a postdoctoral researcher at NYU. He is currently a Changjiang Professor, the Boya Chair Professor, Senior Professor of the Center for Life Sciences and Director of the Institute of Stem Cell Research at Peking University. For a long time, Professor Deng has been committed to using chemical reprogramming to establish the methods, principles, and applications of regulating cell plasticity. He has published more than 100 papers and has been cited more than 16,000 times. In 2019, he was awarded Nature's 10 for his achievements in the field of CRISPR gene editing in human stem cells.
Business Model